Cell and gene therapy is a rapidly evolving field that promises to revolutionize the treatment of various diseases. However, the development and commercialization of these therapies face significant challenges, such as complex and costly manufacturing processes, regulatory uncertainties, and ethical issues. In this article, we will explore how some cell and gene therapy developers are trying to overcome these hurdles by advancing process standards and sharing data.
One of the key challenges in cell and gene therapy development is to demonstrate the safety and efficacy of the gene delivery vectors, which are the vehicles that carry the therapeutic genes into the target cells. These vectors can be derived from viruses, such as adeno-associated virus (AAV), or non-viral methods, such as nanoparticles or plasmids.
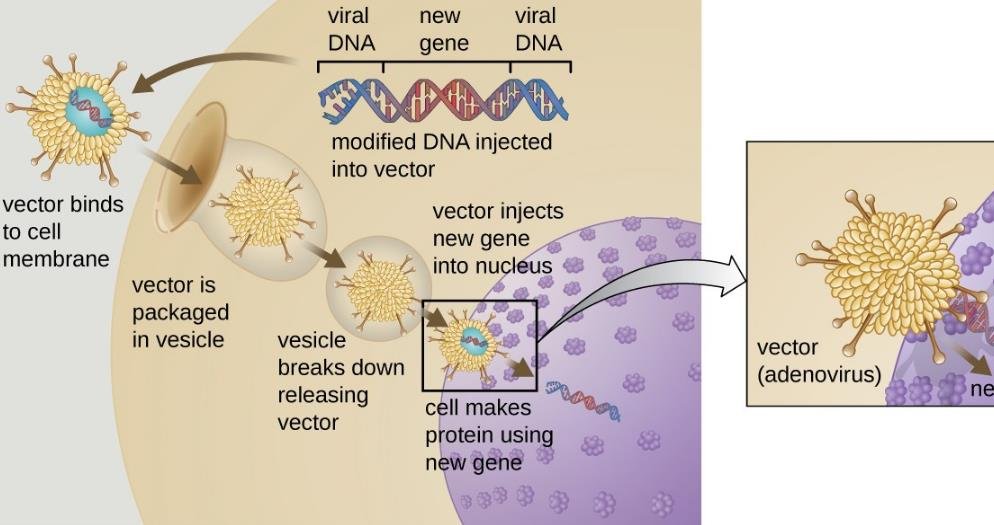
To evaluate the biodistribution of the vectors, which is the distribution of the vectors in the body after administration, the FDA requires animal studies that can be time-consuming, expensive, and ethically questionable. Moreover, the results of these studies may not be predictive of the human response, as different animal species may have different physiological and immunological reactions to the vectors.
To address this issue, some cell and gene therapy researchers are attempting to shape regulatory guidance by identifying well-characterized vector subtypes and dose ranges that can reduce the need for animal studies without increasing the risk to patients. For example, Dr. Terence R. Flotte, dean of the University of Massachusetts Medical School, is an expert on AAV vectors for pediatric genetic diseases. He is part of a group that represents the American Society of Gene and Cell Therapy (ASGCT) on creating platforms to promote AAV vectors in diseases that are not yet being addressed, but which are similar enough to those under investigation.
Dr. Flotte suggests that if a vector subtype, dosage, and administration route have been proven to be safe and effective in one disease, they could be applied to another disease with minimal additional animal studies. This would require a degree of transparency and cooperation among researchers, regulators, and funding agencies, as well as a clear and streamlined packet of preclinical studies that would facilitate parallel development of products for single-gene disorders.
Improving Mouse Models for Gene Integration
Another challenge in cell and gene therapy development is to assess the potential risks of gene integration, which is the insertion of the therapeutic gene into the host genome. This can have unintended consequences, such as disrupting the function of other genes, activating oncogenes, or causing immune reactions. Therefore, the FDA requires mouse models that can mimic the human gene integration patterns and outcomes.
However, these mouse models may not be reliable or relevant, as they may not reflect the actual clinical conditions, such as the vector dose, the target tissue, or the patient population. Furthermore, these models may be costly and labor-intensive, as they require large numbers of animals and long-term follow-up.
To address this issue, some cell and gene therapy researchers are trying to improve the mouse models by using more advanced techniques, such as CRISPR-Cas9, to create more accurate and representative models of human gene integration. For example, Dr. Cynthia E. Dunbar, senior investigator at the National Heart, Lung, and Blood Institute, is an expert on hematopoietic stem cell gene therapy. She is leading a project that aims to generate mouse models that can better predict the integration sites and consequences of different vector types and delivery methods.
Dr. Dunbar hopes that by using CRISPR-Cas9, she can create mouse models that have the same integration sites as humans, as well as the same genetic background and disease phenotype. This would allow her to evaluate the safety and efficacy of different vectors and delivery methods in a more realistic and relevant way, as well as to reduce the number of mouse models and studies required by the FDA.
Conclusion
Cell and gene therapy is a promising and innovative field that has the potential to cure many diseases that are currently incurable or difficult to treat. However, the development and commercialization of these therapies face many challenges, such as complex and costly manufacturing processes, regulatory uncertainties, and ethical issues. To overcome these challenges, some cell and gene therapy developers are trying to advance process standards and share data, in order to streamline regulatory guidance and accelerate clinical translation. By doing so, they hope to bring these therapies to the patients who need them as soon as possible.
